Superphage is a derivative of M13KO7. In bacteria, the wildtype pIII level from M13KO7 is much higher than that of antibody-pIII fusion protein from phagemid. Moreover, phage assembly has a preference for the wild-type pIII even if the wild-type pIII and fusion pIII are at the same level. As a result, the antibody display level is about 1% in an M13KO7-packaged phage library, indicating that 99% of the library is "bald" and doesn't display any antibodies [1-2]. The low display level results in low success rate of isolating specific antibodies [3].
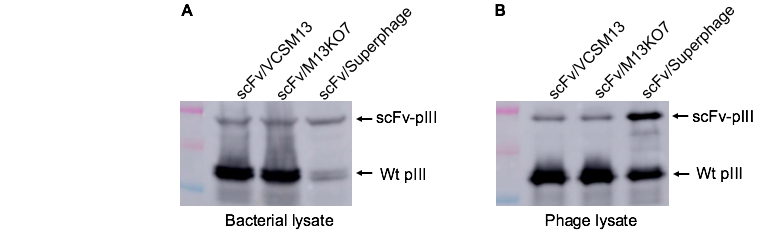
Figure 1. Superphage dramatically decreases wildtype pIII expression and increases scFv display on the phage. (A) TG1 cells were transformed with phagemid pFSA which expressed scFv-pIII fusion protein The cells grow in 2YT media containing 2% glucose and 100ug/ml carbenicillin until OD600 ~0.5. Divide the cell culture into 3 portions, and add VCSM13, M13KO7, and Superphage at a multiplicity of infection (MOI) of 10:1 (phage-to-cells ratio) to the culture respectively. Shake at 60rpm for 15min and then at 250rpm for 45min. Change media to 2YT containing 500ug/ml IPTG and 100ug/ml carbenicillin and 50ug/ml kanamycin. Shake at 250rpm for 5 hours and collect bacteria for western blot with anti-pIII mAb to compare the level of wild-type and fusion pIII. The result showed that Superphage dramatically decreased wild-type pIII expression compared to M13KO7. (B) After overnight culture, phages were purified from bacterial supernatant, and analyzed by western blot with anti-pIII mAb to estimate scFv display level. In Superphage-packaged phages, the level of scFv-pIII fusion protein is about one-third of wildtype pIII. Usually, there are 3-5 copies of pIII on each mature phage, indicating that one copy of scFv-pIII fusion protein is displayed on the surface of each phage averagely. In M13KO7/VCSM13-packaged phages, the scFv display level is much lower.
Mechanism: Superphage dramatically suppresses the expression of wildtype pIII (Figure 1A). The level of wildtype pIII is so low that it is not enough for phage assembly and more antibody-pIII fusion protein is forced to participate in the process. As a result, the antibody display level was increased about 100-fold compared to M13K07-packaged phages. Superphage-packaged phages have the following features: (1) The antibody display level is ~100% (Figure 1B); (2) It is still monovalent display, good for selecting high-affinity antibodies; (3) Each phage has multiple wild-type pIII so that phage infectivity is not compromised; (4) The phage titer is high; about 1011 cfu/ml; (5) In Superphage-packaged phage library, the ratio of phagemid particles to phage particles is 500000:1. In contrast, the ratio is about 10:1 in the M13KO7-packaged phage library; (6) Superphage is compatible with all phagemid and bacterial strains, unlike Phaberge, EX-phage, and Ex12 which works only in the amber non-suppressor strains.
The coupling of phenotype and genotype is not always true [4]. During a library assembly, the displayed protein may be either wild type (derived from the helper phage) or antibody-pIII fusion protein (derived from the phagemid), and the packaged genome may be either phage or phagemid. Therefore, there are four kinds of virus particles in an antibody phage library. (i) phagemid genome and antibody-pIII fusion protein (the desired result). (ii) phagemid genome and wild type pIII (unable to bind the target and will be washed away during the panning). (iii) phage genome and antibody-pIII fusion protein (possible to bind a target but cannot be amplified). (iv) phage genome and wild type pIII (the helper phage itself). In theory, the mutated packaging signal in helper phage should significantly reduce the number of helper phage particles during the library assembly. However, the number of phage particles can sometimes equal, or exceed, the number of phagemid particles, which can significantly compromise subsequent selections. The ratio of phagemid particles to phage particles depends on both helper phage and phagemid vector. In pFSA/Superphage system, the ratio of phagemid particles to phage particles was 500000:1 (Table 1), and almost all the virus particles are phagemid particles.
Table 1. Comparison of features of helper phages.
|
|
Hyperphage |
M13KO7/VCSM13 |
Superphage |
|
WT pIII level |
Zero |
High |
Low |
|
Display level |
100% |
1% |
100% |
|
Monovalent |
No |
Yes |
Yes |
|
Affinity |
Low |
High |
High |
|
Titer |
Low |
High |
High |
|
Phagemid particles: phage particles |
100:1 |
7:1 |
500000:1 |
Table 2. Specifications of Superphage
Vector category | Helper phage |
Specificity | Infection of bacteria via pIII |
Quantity | 2.0E+12 cfu |
Selection | Kanamycin |
Reconstitution | Spin down briefly. Add 1.0ml of H2O, shake at 37°C for 30min until it is dissolved completely. Add 0.8 ml of glycerol, and mix. The final concentration is 1.0E+12 cfu/ml. |
Storage | After reconstitution, stored at -20°C |
Expiration | Lyophilized, 3years; Reconstituted, 1year. |
References
1. Kramer RA, Cox F, van der Horst M, et al. A novel helper phage that improves phage display selection efficiency by preventing the amplification of phages without recombinant protein. Nucleic Acids Res. 2003; 31(11): e59.
2. Azzazy HM, Highsmith WE Jr. Phage display technology: clinical applications and recent innovations. Clin Biochem. 2002; 35(6): 425-45.
3. Baek H, Suk KH, Kim YH, et al. An improved helper phage system for efficient isolation of specific antibody molecules in phage display. Nucleic Acids Res. 2002; 30(5): e18.
4. Chasteen L, Ayriss J, Pavlik P, et al. Eliminating helper phage from phage display. Nucleic Acids Res. 2006; 34(21): e145.
Superphage amplification
1. XL10-Gold was cultured overnight at 30°C in 2xYT containing 10 ug/ml tretracycline.
2. 10ml 2xYT (containing 10ug/ml tretracycline) + 200ul overnight culture. Shaking at 250rpm and 30°C until OD600 =0.3 (about 3hours).
3. Add Superphage at a multiplicity of infection of 20:1 (phage-to-cell-ratio).
4. Shake for 15min at 60rpm and 30°C, and then for 45min at 250rpm and 30°C.
5. Add the 10ml of infected culture to 1000 ml of prewarmed 2xYT containing 50ug/ml kanamycin, shake at 250rpm and 30°C until bacterial growth to the stationary phase (~24hours).
6. Purify Superphage from the supernatant by PEG/NaCl precipitation.
Phage purification (PEG/NaCl precipitation)
1. 12000xg, 10min at 4°C degree, collect the supernatant and trash the pellet.
2. Add 40g of PEG-8000 (4% w/v) and 30g of NaCl (3% w/v). After the solid is completely dissolved, keep in ice for 60min or overnight at 4°C.
3. Spin at 12000g for 20min at 4C. Discard the supernatant; spin down again and remove the remaining liquid.
4. Resuspend the phage pellet in 20ml of H2O containing 40% glycerol and 1% BSA.
5. 15000xg for 10min at 4°C, keep the liquid and trash the pellet.
6. Aliquot and store at -20°C.
Titration
1. XL10-Gold was cultured overnight at 30°C in 2xYT containing 10 ug/ml tretracycline.
2. 5ml 2xYT (containing 10ug/ml tretracycline) + 100ul overnight culture. Shaking at 250rpm and 30°C until OD600 around 0.5 (about 3hours).
3. Infect the E coli with serially diluted phage and make sure the cell number is greater than phage number.
4. Shake for 15min at 60rpm and 30°C, and then for 45min at 250rpm and 30°C.
5. Spread the culture on kanamycin plates, and put the plates at 37°C overnight.
6. Count the colony number on the kanamycin plates, and calculate the cfu/ml of phage particles.
- The product is available to nonprofit organizations or for-profit companies.
- Buyers are NOT allowed to transfer or resell this product in any form.
Superphage
- Product Code: HP001
-
$2,098.00
Related Products
pFSA
pFSA is a phagemid vector designed for high-level expression and display of antibody fragment-p..
$1,098.00
M13PK
M13PK is the double-stranded, circular DNA derived from bacteriophage M13 phage, and has the fo..
$598.00
Phage Display Package
The phage display package contains 4 items. 1) pFSA is a phagemid vector designed for high-leve..
$3,098.00




