Bispecific T cell engagers (BiTEs) are fusion proteins consisting of two antibody fragments, one binds to T cells via the CD3 receptor, and the other to tumor cells via tumor-specific molecules. BiTEs form a link between T cells and tumor cells, which promotes T cell-mediated lysis of tumor cells via releasing perforin and granzymes. Although BiTE therapy is very promising, it can induce the systemic release of proinflammatory cytokines leading to cytokine release syndrome (CRS). T cells possess a dual activation threshold (one for cytotoxicity and the other for cytokine production)[1]. T cell killing does not require the formation of a stable mature immunological synapse, and a low-affinity anti-CD3 can induce tumor killing with minimal cytokine release [2-4].
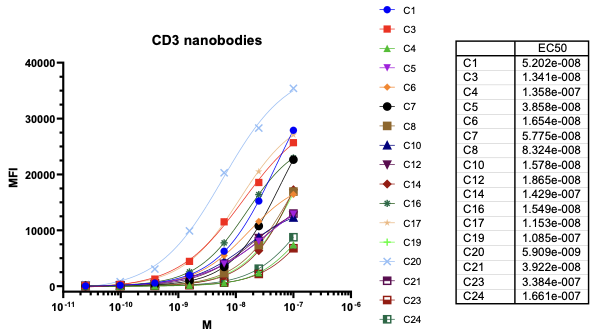
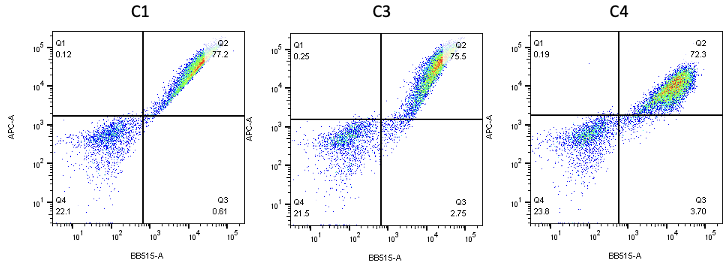
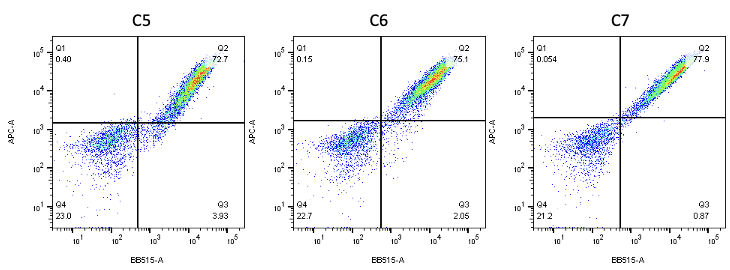
Nanobodies are the next generation of cancer therapeutics[5-6]. CD3 nanobodies may be the perfect choice for making BiTEs[7-8]. 17 human CD3 nanobodies are isolated from synthetic and humanized nanobody libraries[9-11]. They were initially characterized and fully sequenced. The affinity scale ranges from uM to nM. If you are interested in the further development or have any questions, please contact us: info@phageomics.com or antibodydisplay@gmail.com.
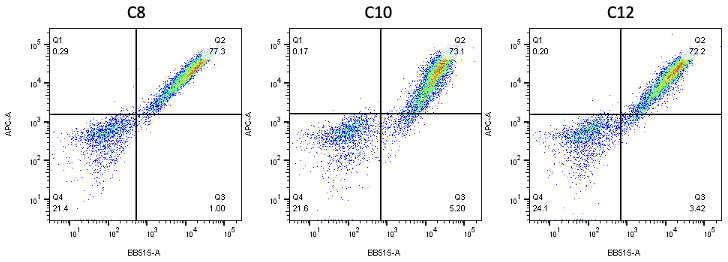
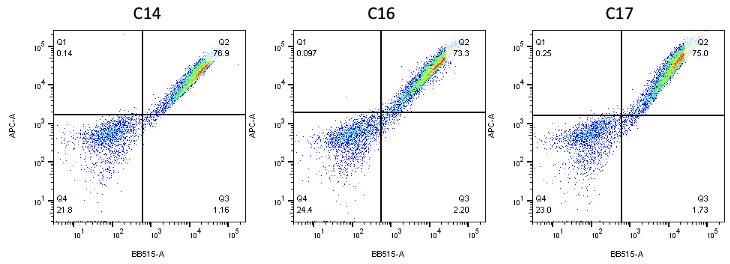
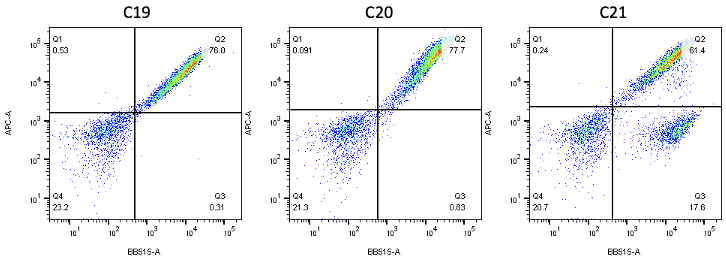
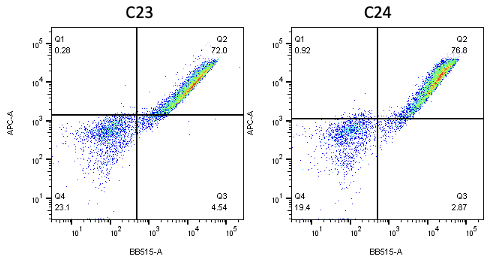
References
1. Faroudi M, Utzny C, Salio M, et al. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. PNAS 2003; 100: 14145–50.
2. Dang K, Castello G, Clarke SC, et al. Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release. J Immunother Cancer. 2021; 9(6): e002488.
3. Purbhoo MA, Irvine DJ, Huppa JB, et al. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004 May;5(5):524-30
4. Trinklein ND, Pham D, Schellenberger U, Buelow B, et al. Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies. MAbs. 2019; 11(4):639-52.
5. Yang EY, Shah K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front Oncol. 2020; 10: 1182
6. Bannas P, Hambach J, Koch-Nolte F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics. Front Immunol. 2017; 8: 1603
7. Moradi-Kalbolandi S, Sharifi-K A, Darvishi B, et al. Evaluation the potential of recombinant anti-CD3 nanobody on immunomodulatory function. Mol Immunol. 2020; 118: 174-81.
8. Khatibi AS, Roodbari NH, Majidzade-A K, et al. In vivo tumor-suppressing and anti-angiogenic activities of a recombinant anti-CD3ε nanobody in breast cancer mice model. Immunotherapy. 2019; 11(18): 1555-67
9. Zimmermann I, et al. Synthetic single domain antibodies for the conformational trapping of membrane proteins. Elife 2018; 7: e34317.
10. Pardon E, et al. A general protocol for the generation of Nanobodies for structural biology. Nat Protoc. 2014; 9(3): 674-93.
11. Moutel S, et al. NaLi-H1: A universal synthetic library of humanized nanobodies providing highly functional antibodies and intrabodies. Elife. 2016; 5: e16228
human CD3 nanobodies
- Product Code: nanocd3
-
$0.00