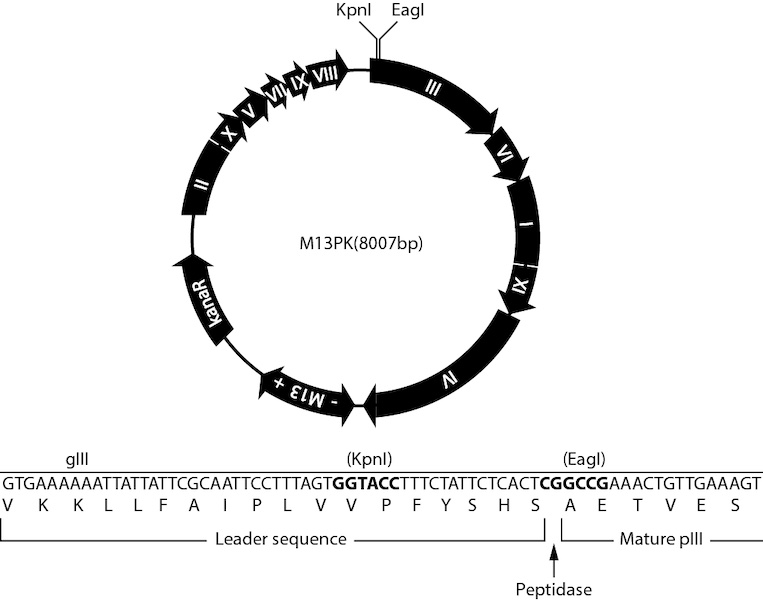
M13PK is the double-stranded, circular DNA derived from bacteriophage M13 phage, and has the following features.
- Kanamycin resistance gene was introduced for positive selection and ease of titration.
- Cloning sites at 5´ end of gene III are for display of peptide-pIII fusion protein.
- The sequences were optimized to reduce repeats and increase DNA stability.
- It a phage vector, not a phagemid vector. All 5 copies of pIII on each virion will be fused to the cloned peptide. Peptides longer than 30 amino acids have a deleterious effect on the infectivity function of pIII, so this vector is suitable for the display of short peptides.
Specifications
Vector type | Phage vector |
Replication origin | M13 |
Selection | Kanamycin |
Cloning sites | KpnI, EagI |
DNA size | 8007bp |
Quantity | 20ug, Lyophilized |
Reconstitution | Spin down, add 40ul of nuclease-free water/TE/EB buffer, shaking for 30min at 50°C until completely dissolved. Spin down again. |
Storage | After reconstitution, Store at -20°C |
Map of M13PK
Sequences are available upon order and request.
Preparation of M13PK double-stranded DNA
- Culture TG1 or SS320 in 200ml of 2xYT medium containing 0.1% glucose at 37℃ and 250rpm OD600 = 0.8 to 1.0.
- Infect the cells with phage M13PK at multiplicity of infection of 20:1 (phage-to-cell-ratio) for 15 min at 37℃.
- Add 200ul of 15 mg/ml chloramphenicol in ethanol (final concentration, 15 μg/ml) to the culture. Incubate the culture an additional 2 hr.
- Centrifuge 10 min at 6000g to harvest the cells. Prepare double-stranded DNA using TaKaRa Maxiprep kit (# 740414.50)
Amplification of M13PK phage
- XL10-Gold was cultured overnight at 30°C in 2xYT containing 10 ug/ml tetracycline.
- 10ml 2xYT (containing 10ug/ml tetracycline) + 200ul overnight culture. Shaking at 250rpm and 30°C until OD600 =0.3 (about 3hours).
- Add M13PK phage at a multiplicity of infection of 20:1 (phage-to-cell-ratio).
- Shake for 15min at 60rpm and 30°C, and then for 45min at 250rpm and 30°C.
- Add the 10ml of infected culture to 1000 ml of prewarmed 2xYT containing 50ug/ml kanamycin, shake at 250rpm and 30°C until bacterial growth to the stationary phase (~24hours).
- Purify M13PK phage from the supernatant by PEG/NaCl precipitation.
Cloning strategy
- KpnI and EagI digestion of M13PK DNA, and gel purification.
- PCR amplification of insert with forward primer 5’-TTT AGT GGT ACC TTT CTA TTC TCA CTC G NNN NNN NNN NNN-3’ and reverse primer 5’- AAC AGT TTC GGC CGA NNN NNN NNN NNN-3’.
- KpnI and EagI digestion of the insert, and gel purification.
- Ligation of KpnI and EagI digested M13PK and insert.
- DNA purification by ethanol precipitation.
- Transformation of TG1 competent cells.
Production of peptide phage library
- Add SOC to the transformed cells and incubate for 60 minutes at 30°C and 250rpm.
- Add 2xYT containing 50ug/ml kanamycin to the culture and incubate overnight at 30°C and 250rpm.
Phage purification
- 10000xg, 10min at 4C degree, collect the supernatant and trash the pellet.
- Add 40g of PEG-8000 (4% w/v) and 30g of NaCl (3% w/v). After the solid is completely dissolved, keep in ice for 60min or overnight at 4°C.
- Spin at 15000g for 15min at 4C. Discard the supernatant; spin down again and remove the remaining liquid.
- Resuspend the phage pellet in 25ml of TBS containing 0.5% BSA and 0.04% of NaN3.
- 20000g for 10min at 4°C to clear the supernatant.
- Add 25ml of glycerol and mix completely. Store at -20°C for 2 years and at -80°C for long term.
Titration
- XL10-Gold was cultured overnight at 30°C in 2xYT containing 10 ug/ml tetracycline.
- 5ml 2xYT (containing 10ug/ml tetracycline) + 200ul overnight culture. Shaking at 250rpm and 30°C until OD600 around 0.5 (about 3hours).
- Infect the E coli with serially diluted phage and make sure the cell number is greater than phage number.
- Shake for 15min at 60rpm and 30°C, and then for 45min at 250rpm and 30°C.
- Spread the culture on carbenicillin and kanamycin plates respectively, and put the plates at 37°C overnight.
- Count the colony number on the carbenicillin and kanamycin plates, and calculate the cfu/ml of the phagemid particles and phage particles respectively.
- This product is available to nonprofit organizations or for-profit companies.
- Buyers are NOT allowed to transfer or resell this product in any form.
M13PK
- Product Code: PV001
-
$598.00
Related Products
pFSA
pFSA is a phagemid vector designed for high-level expression and display of antibody fragment-p..
$1,098.00
Superphage
Superphage is a derivative of M13KO7. In bacteria, the wildtype pIII level from M13KO7 is much highe..
$2,098.00
Phage Display Package
The phage display package contains 4 items. 1) pFSA is a phagemid vector designed for high-leve..
$3,098.00